Vaccine preference a problem for EU leaders
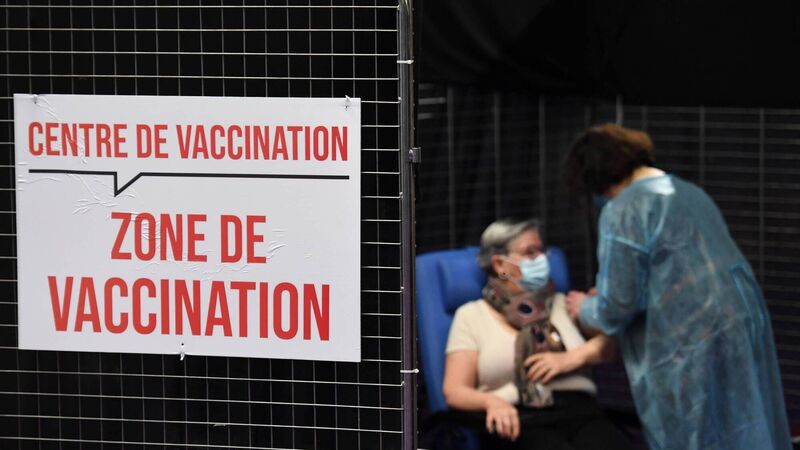
A woman receives a dose of the Pfizer-BioNtech Covid-19 vaccine at a vaccination centre in Garlan, western France, on Tuesday. Picture: Fred Tanneau/Getty Images
The scientist in charge of France’s vaccine rollout, Alain Fischer, must sometimes feel like Sisyphus, forever rolling his boulder up the hill.
After months of patiently working to win over a doubting public’s acceptance of the groundbreaking mRNA vaccines from Pfizer and Moderna, he’s now fighting to convince French doctors to offer AstraZeneca’s more traditional shot to their patients.












