Modulating the gut microbiome could represent a new frontier for cancer medicine
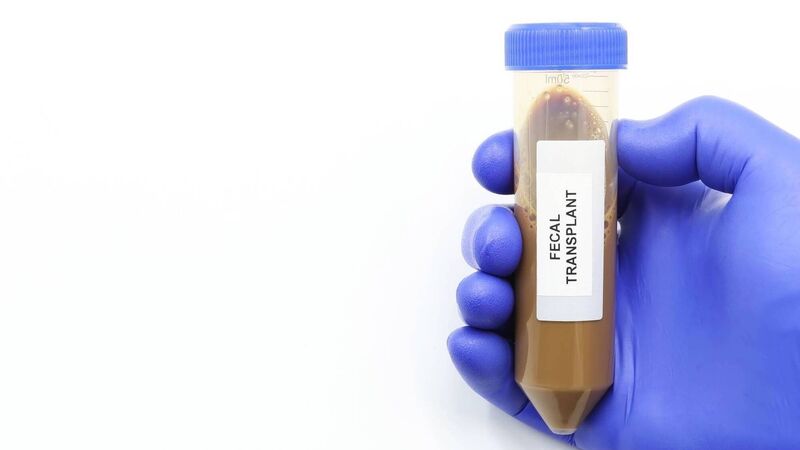
Fecal transplant or fecal matter transplant (FMT). A laboratory tube with with fecal microbiota to cure persistent digestive diseases such as infection by Clostridium bacteria











