HSE drafting in army personnel to administer vaccine amid capacity concerns
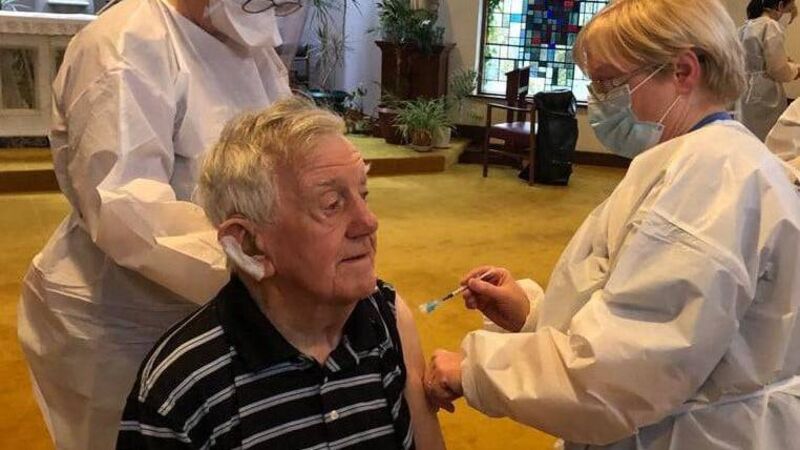
A resident of Maypark House nursing home in Waterford receives his vaccination
The HSE is in talks with the Defence Forces about drafting in army personnel to administer Covid-19 vaccine and alleviate mounting concerns over capacity issues.
News of the discussions comes as it has emerged the European Medicines Agency expects drug maker AstraZeneca to apply for approval of its Covid-19 vaccine next week with approval hopefully granted by the end of the month.










