Up to 80,000 people in Ireland may qualify for State-funded weight-loss injections
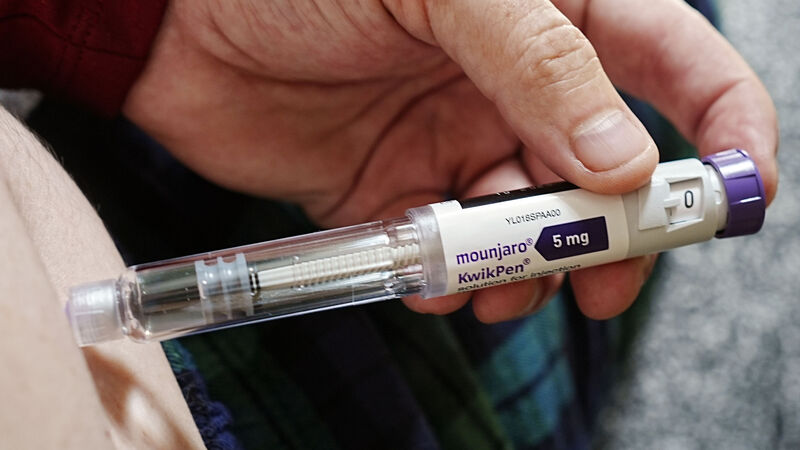
Around 80,000 of the 1.2m people classified as obese in Ireland will likely avail of State-funded weight-loss injections if the drugs are approved for medical card holders or under the HSE's Drug Payment Scheme.
The HSE's national clinical lead for obesity, Professor Donal O’Shea, says a health technology assessment (HTA)— evaluating the medical, social, ethical, and economic implications — of the weight-loss wonder-drug Mounjaro is to be published in the coming weeks, which is the “precursor to making a decision about who will get it”.
Currently, similar drugs are provided under public schemes for people with diabetes, but several manufacturers have been granted permission by the European Medicines Agency (EMA) for them to be used for weight loss treatment.
The companies are seeking HSE reimbursement that would potentially make the drugs free for medical card holders or capped at €80 per month under the Drug Payment Scheme.
Danish pharmaceutical company Novo Nordisk applied for HSE reimbursement for its Wegovy injection, for adults and adolescents, in March 2025.
As the drug is not yet covered by the public scheme for weight-loss patients, Wegovy is listed, for the first three dosages, at €240 per month on one pharmacy website, increasing to €320 and €355 for higher dosages.
Eli Lilly has applied for HSE reimbursement for Mounjaro for both weight-loss treatment and diabetes.
With a valid prescription, several Irish pharmacies currently list Mounjaro’s starting price at €275 per month. This rises to as much as €595 per month for the highest required dose.
Health minister Jennifer Carroll MacNeill said the application for Mounjaro under the reimbursement scheme could be approved by the end of the year.
Prof O’Shea told the that a HTA on Mounjaro will likely limit who can initially get the injections due to the huge costs involved for the HSE of providing the treatment.
“I'm concerned about the lengths people are having to go to access what are effective, safe treatments,” Prof O’Shea said.
“The HSE is about to publish its HTA on Mounjaro. This is the precursor to making a decision about who will get it.
“It's likely to come in somewhere quite along the lines of its availability in the UK, which is for people with a BMI over 35, plus complications.
“That will have it available to maybe 80,000 people in Ireland. The bottom line is you have 25% of the population living with obesity. That's 1.2m people.
“They simply can't begin by letting it be open to everybody, just on a cost basis.
“We have to make sure we start using it in, initially, the high-risk group and then widen out its use.”
Prof O'Shea added that he “desperately” wants the treatments available, but added that it has to be “brought in at a point where it won’t break you financially”.












