Mylan to sell generic EpiPen amid US costs outcry
The offer by the drugmaker, which has significant facilities across Ireland, to help patients cover out-of-pocket expenses was criticised as insufficient.
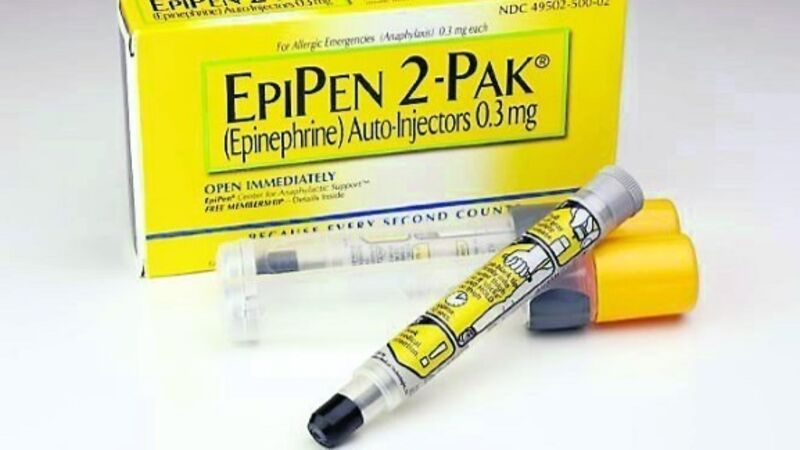
It will introduce a generic EpiPen identical to the branded product, which includes the drug itself and the handheld pen with a needle that injects the shot.













