Court clears inspectors in implants case
In a statement the Aix-en-Provence appeal court said the German agency had “respected the obligations incumbent upon it as a certifying organisation”.
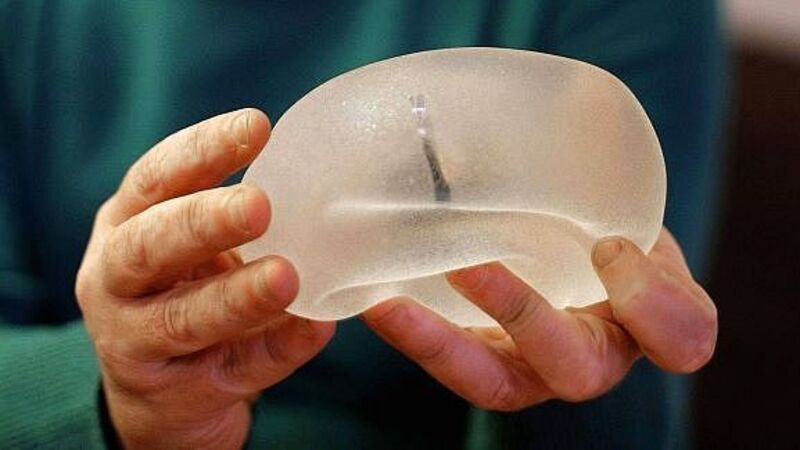
Poly Implant Prothèse (PIP), the French company at the centre of the scandal, sold implants globally, incluing hundreds in Ireland, over almost two decades until investigators discovered it was passing off industrial silicone as a much pricier medical product.














