Have your say: EMA approves Pfizer jab for children aged 5 to 11
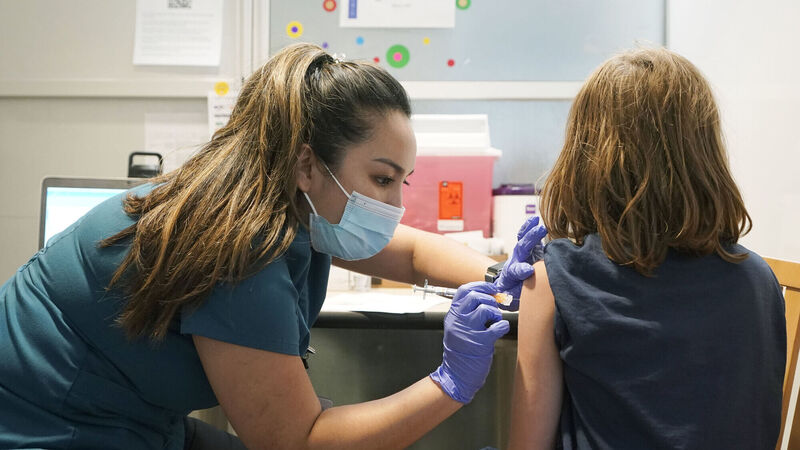
Courtney Martin, left, a nurse at the University of Washington Medical Center, gives the first shot of the Pfizer COVID-19 vaccine to Ani Hahn, 7, Tuesday, Nov. 9, 2021, in Seattle. Picture: AP Photo/Ted S. Warren
The European Medicines Agency (EMA) has approved the Pfizer/BioNTech Covid-19 vaccine for us for children between the ages of 5 and 11-years.
Both the US and Canada have already authorised the use of Pfizer's Covid-19 vaccine for children in the age grouping.












