Concerns raised over Silimed breast implants
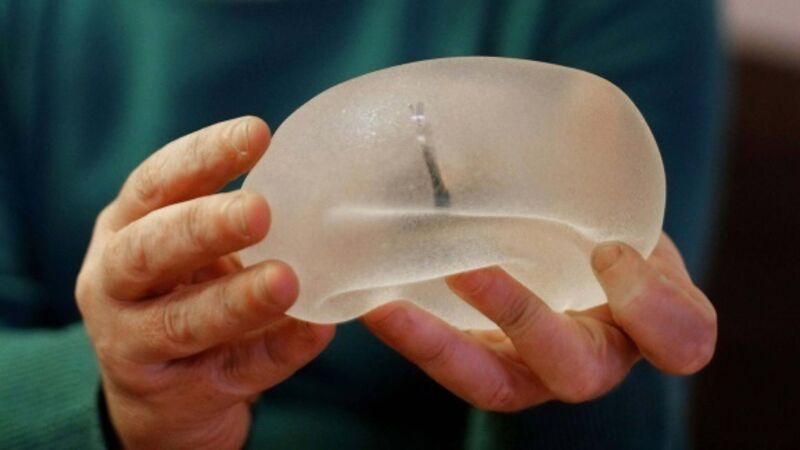
Ireland’s Health Products Regulatory Authority (HPRA), along with its fellow European medical device regulators), has been told that the CE quality conformity certificate for all medical devices made by the Brazilian manufacturer Silimed has been suspended.
The move comes after an inspection of the company’s manufacturing plant in Brazil found the presence of particles on the surface of some of the devices.













