CASE STUDIES: Concerns over cervical cancer vaccine in Ireland
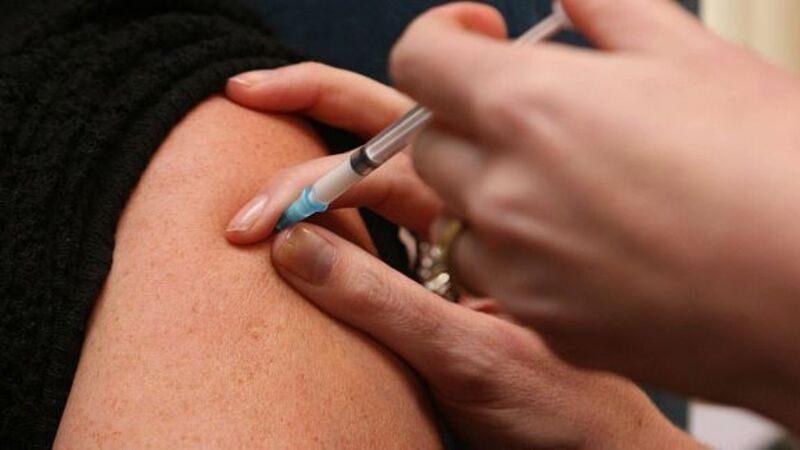
Gardasil, the vaccine manufactured by Sanofi-Pasteur, is offered to girls from the age of 12 to protect them against developing certain strains of cervical cancer.
Speculation about potential adverse reaction to the vaccine has grown internationally, and this week, the European Medicines Agency launched a review of the cervical cancer vaccine’s safety, while asserting that the review should not raise questions about whether the vaccine’s benefits in preventing cervical cancer outweigh their risks.













