EU to look at research options on narcolepsy
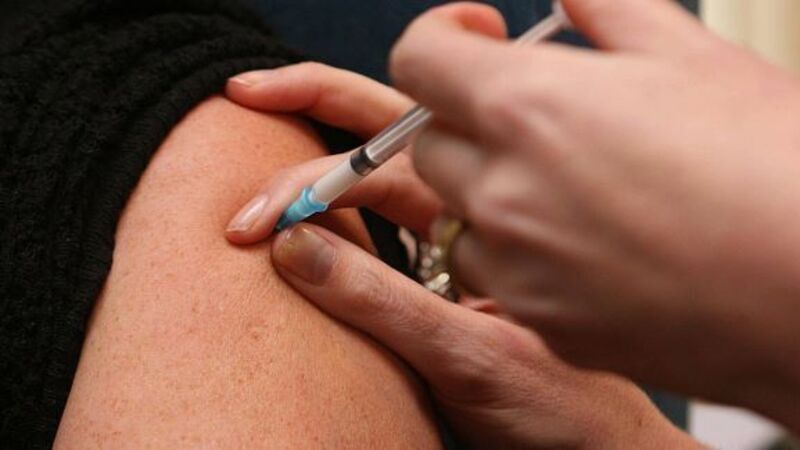
Within three weeks, Ellen, a twin, started falling. When she began to lose consciousness in the middle of meals, they knew something was wrong.
Like millions of others, Ellen received Pandemrix, a drug manufactured by GlaxoSmithKline and approved by the European Medicines Agency for treating swine flu.













