US delays approval of Eli Lilly Alzheimer's treatment
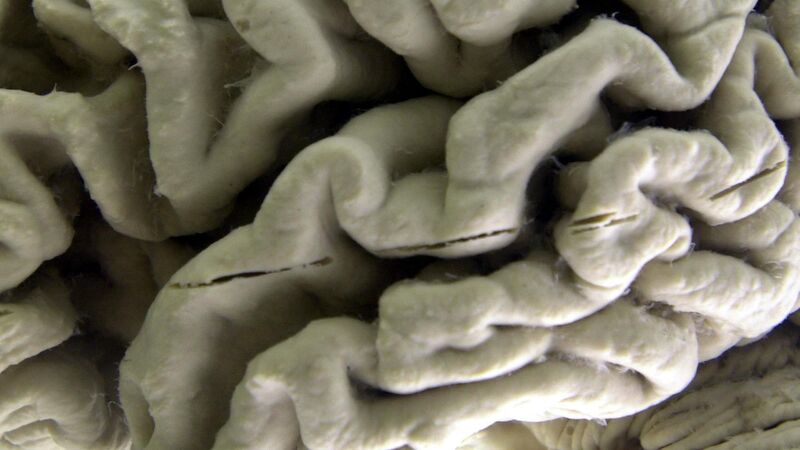
Donanemab, given by infusion once a month, is designed to clear from the brain a sticky protein called amyloid tied to Alzheimer's disease.
The US Food and Drug Administration has delayed its decision on Eli Lilly's experimental treatment for early Alzheimer's disease and will hold a meeting of outside experts to discuss its safety and efficacy, the company said.
This is the second regulatory delay for the drug, donanemab, after Lilly released clinical trial data last year that showed the treatment was safe and effective. Lilly said no date has been set yet for the advisory committee meeting to discuss the medicine, but it could be several months before it is held.












