J&J gets earnings boost from blockbuster psoriasis drug
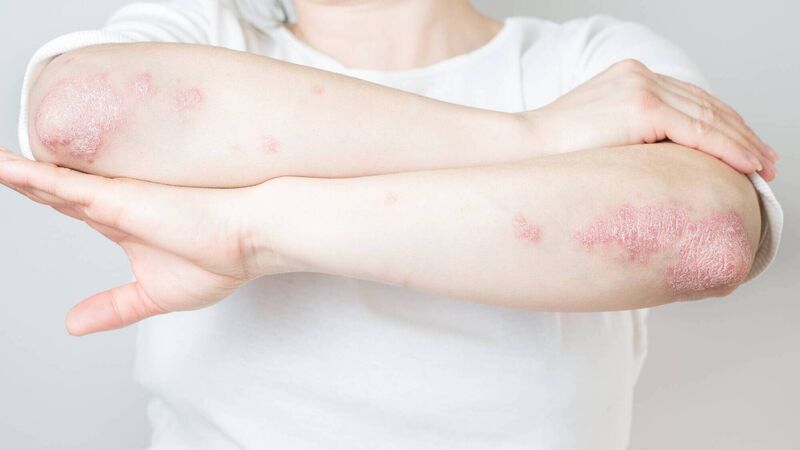
A key patent for J&J's Stelara psoriasis expired in the US last year.
Johnson & Johnson (J&J) has posted quarterly results just above expectations, helped by strong sales of its blockbuster psoriasis drug Stelara.
A key patent for Stelara expired in the US last year, but J&J struck deals with competitors to delay the launches of their biosimilars until 2025. Amgen will be the first to launch its near-copy, Wezlana, next year.











