The latest on 16 vaccines in the global race for Covid-19 cure
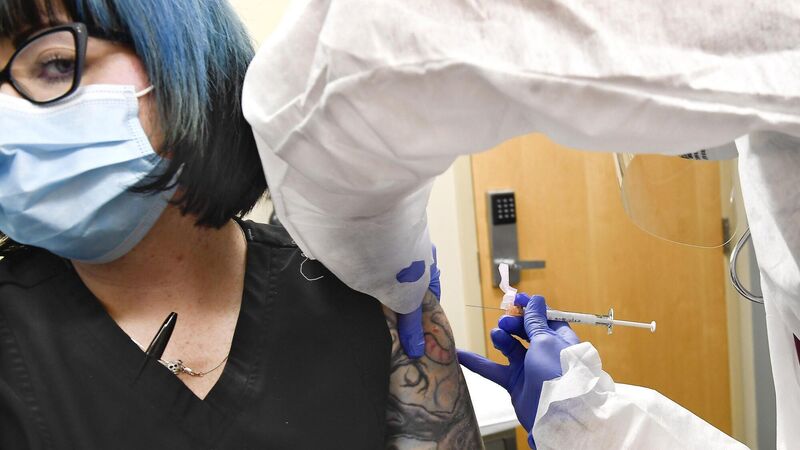
Volunteer Melissa Harting receiving an injection of mRNA-1273, the candidate in Moderna's trial in New York. Picture: AP/Hans Pennink
The race to secure a Covid-19 vaccine is back on track after two key trials were restarted after they had been paused due to safety concerns.
The vaccines could transform the global economy some time next year if they work. Here's the current state of play on vaccines and treatments.
's BNT162 programme is a messenger RNA vaccine platform that the German company is developing with Pfizer. In China, BioNTech is co-developing vaccines with Shanghai Fosun. European regulators have begun a real-time review of a phase three trial from BionTech. Pfizer expects the company could file for an emergency use authorisation in the US before the end of November.
's mRNA-1273 uses messenger RNA to prompt the body to make a key protein from the virus, creating an immune response. The programme is backed by $955m (€820m) from the US government, which separately reached a $1.5bn deal for 100m doses. Moderna has recruited 30,000 patients for a trial in collaboration with the National Institutes of Health in the US.
and resumed late-stage trials in the US weeks after a pause due to a volunteer's illness in a separate UK study which has also restarted. European regulators have begun a real-time review of a UK trial. The vaccine they are testing is made from a harmless virus that’s been altered to produce the surface spike protein from SARS-CoV-2.
has a phase three trial on an unnamed adenovirus-based vaccine as well as two backups. The company has clinched supply deals with the US and the EU.
and have two unnamed candidates in phase three. Hundreds of thousands of people, including frontline medical workers, have already received China Biotec's shots. It is in final-stage trials in countries across the Middle East and South America and it is expected that the shots will be available in China as soon as end-2020.

says its vaccine spurred the creation of neutralising antibodies in more than 90% of people tested in early-stage trials, and is safe in older adults. a vaccine which uses inactivated virus in a phase three trial.
The shot was approved in China for emergency use for doctors, customs officials, and other frontline workers, and is simultaneously being put through phase three trials in multiple other countries.
in Russia has a candidate which is a viral vector vaccine based on human adenovirus — a common cold virus —fused with the spike protein of Sars CoV-2 to stimulate an immune response. The shot produced an immune response in early-stage test participants, without adverse safety effects, according to a peer-reviewed study. There is a deal for clinical studies and to supply 100m doses in India.
has spliced the virus’s spike protein into a safe, nonreplicating version of a human adenovirus for its candidate vaccine which is in phase three testing. CanSino has received regulatory approval to start final-stage trials in Russia. The shot has already received a special authorisation to be used by China's military.
's candidate in phase two is an experimental vaccine that uses DNA to activate a patient's immune system. The US Food and Drug Administration had questions about the device to be used to deliver the shot.
's phase two vaccine consists of synthetic spike proteins grown in armyworm moth cells. Novavax has received $1.6bn from the US government as it prepares for a final-stage study this fall. In August, phase two trials began in the US and elsewhere.
and 's candidate is in phase two to develop a single-dose messenger RNA vaccine. Results from a phase one study are expected in the fourth quarter.
and 's unnamed candidate is working on a Sanofi vaccine using technology already employed in one of its flu vaccines, which could speed development and production. In September, Sanofi and Glaxo launched human studies at sites across the so-called Operation Warp Speed programme in the US. The EU and UK have also clinched deals.
has two vaccine candidates in phase one trials that employ existing technology behind a measles virus vector platform discovered by the Institut Pasteur and its own Ebola shot, respectively.
's unnamed candidate is in a phase one trial. When injected, the RNA vaccine candidate delivers genetic instructions to muscle cells to make the "spike" protein on the surface of the coronavirus.
's unnamed candidate is in phase one and, like those from Moderna, Pfizer, and BioNTech, CureVac's shot packages coronavirus-fighting messenger RNA in tiny particles that look to the body like cholesterol. The German government bought a $344m stake in the biotech company in June. In August, it announced a preliminary plan to supply up to 405m doses to EU states.
of China, , and have Clover's phase one shot, which grows proteins resembling the virus’s spike protein in a mammalian cell culture.
—





